ADPS™
KRAS Mutation Test Kit
Ultra-Sensitivity Enhancing KRAS Research Outcomes
Ultra-Sensitivity Enhancing KRAS Research Outcomes
KRAS mutations are observed in various cancers, including colorectal cancer (CRC), non-small cell lung cancer (NSCLC), and pancreatic ductal adenocarcinoma (PDAC). The KRAS protein is a GTPase and one of the key molecules in the signaling pathway downstream of the epidermal growth factor receptor (EGFR). KRAS mutations are mainly found in codons 12, 13, and 61 of exons 2 and 3 , causing alterations in the growth signaling of the p21-ras protein. Most occur in codon 12 or 13, while codon 61 mutations are rare. These KRAS mutations are found in approximately 40% of colorectal cancers, 30% of non-small cell lung cancers, and 90% of pancreatic ductal adenocarcinomas.
In colorectal cancer, Erbitux (cetuximab) and Vectibix (panitumumab) are approved drugs that target EGFR. The presence of KRAS mutations affects the response to treatment with cetuximab and panitumumab, often leading to a negative response in CRC patients. Therefore, detecting KRAS mutations may help guide the treatment of CRC patients.
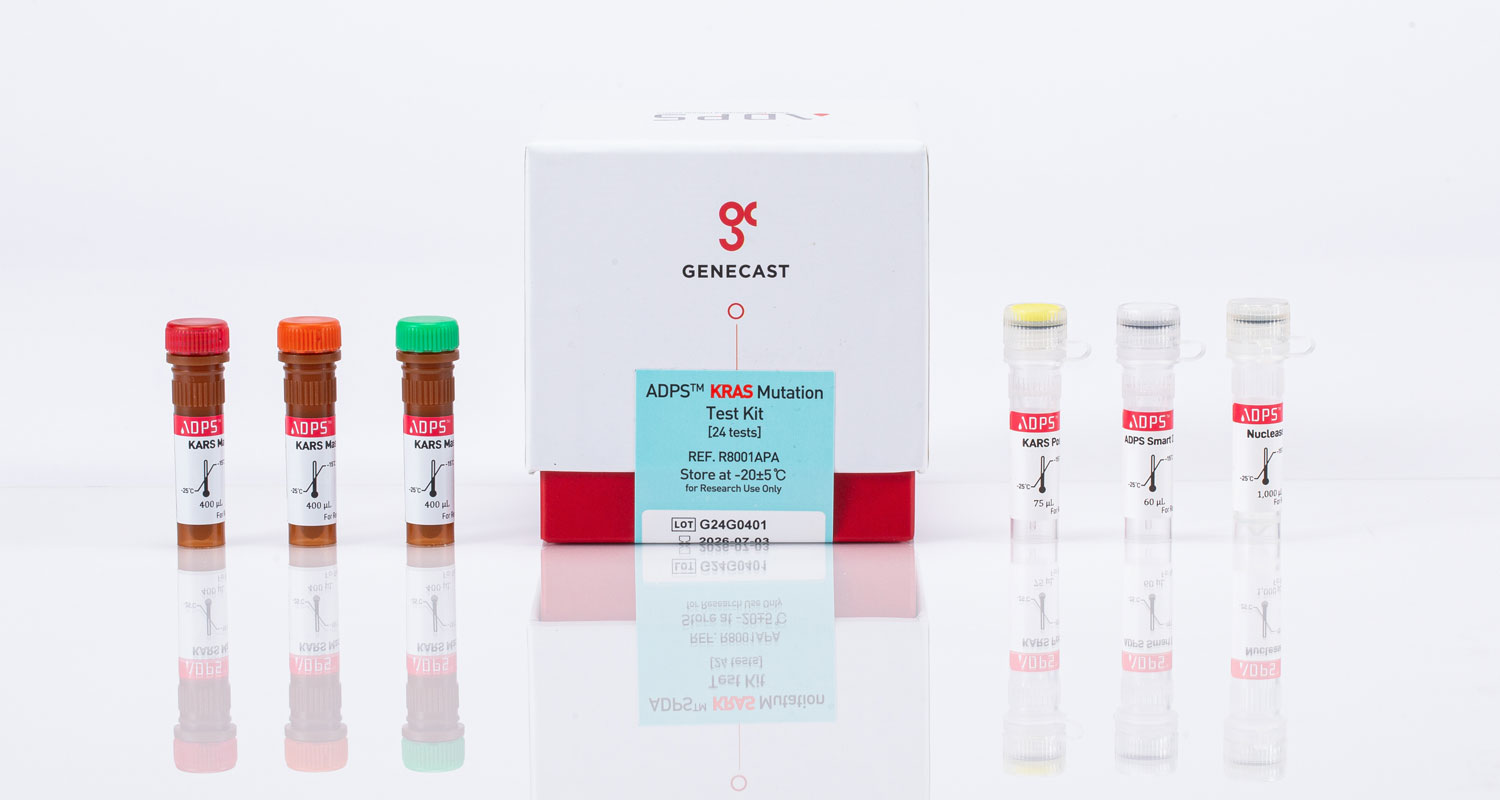
The ADPS™ KRAS Mutation Test Kit detects 19 mutations in exons 2 and 3 of the KRAS gene with a sensitivity of 0.1%. It is designed for testing both tissue and plasma samples with a single kit.
This product is for research use only (RUO) and not intended for use in diagnostic procedures.
| Catalog No. | (RUO) R8001APA |
|---|---|
| Detection target | 19 mutations in KRAS exon 2 and 3 (codon 12, 13 and 61) |
| Assay type | qPCR |
| Sample type | DNA extracted from FFPE, frozen, and fresh cancer tissue or liquid biopsy |
| Master Mixture (MMX) |
3MMXs |
| Internal Control | KRAS intron 4 |
| Detection Sensitivity | 0.1% |
| Detection Specificity | ≥ 99.0% |
| Precision (% CV) | ≤ 5% (CV, coefficient of variation) |
| Storage | -20±5℃ |
| Stability | 18 months |
| Freeze/Thaw stability | 5 times |
| Compatible Instruments | AB 7500 Fast, CFX96, QuantStudio5 |
ADPS™ Oncogene Mutation Test